2019-nCoV Ag Rapid Test (Immunochromatography) Precautions
Intended Use
The kit is used for qualitative detection of 2019-n coronavirus
antibody collected from human nasal swab samples.
As a new product, 2019-nCOV is a kind of β-COVs. Can cause viral
pneumonia, the main clinical manifestations are fever, fatigue and
dry cough. A few patients have symptoms such as nasal congestion,
runny nose, sore throat and diarrhea. Critical patients often
suffer from dyspnea and/or hypoxemia after one week, and severe
patients will rapidly develop into acute respiratory distress
syndrome, septic shock, refractory metabolic acidosis and
coagulation dysfunction.
This product uses lateral flow immunoassay to qualitatively detect
2019-n coronavirus antibody in nasal swabs of suspected patients.
In the acute stage of infection, antigens are usually detected in
nasal swab samples. The positive results indicate the presence of
viral antigen, but the clinical correlation between the patient's
medical history and other diagnostic information is also a
necessary condition to determine the infection status. Positive
results can not rule out bacterial infection or simultaneous
infection with other viruses.
This kit is for laymen to use at home in non-laboratory environment
(such as personal home or some non-traditional places, such as
offices, sports events, airports, schools, etc.). The test results
of this kit are for clinical reference only. It is suggested that
the patient's condition should be comprehensively analyzed
according to the clinical manifestations and other laboratory
tests.
Principle of Detection
This kit is an immunoassay method based on the principle of double
antibody sandwich technology. As an indicator mark, the 2019nCoV
monoclonal antibody with marker was sprayed on the binding pad. In
the detection process, the 2019-nCoV antibody in the sample was
combined with the labeled 2019-nCoV monoclonal antibody to form an
Ag- antibody complex. The complex migrates upward on the membrane
by capillary effect until it is captured by another 2019-n
coronavirus monoclonal antibody pre-coated on the test line,
forming a sandwich complex. If there is 2019-nCoVAg in the sample,
a red band will appear in the T area of the explanation window.
Otherwise, it will be a negative result. Control line (c) is used
for program control. If the test process is executed correctly, it
should always be displayed.
Main Components
The kit consists of a test card, sample buffer and swab.
Test card: It consists of aluminum foil bag, desiccant, test strip
and plastic card. The test strip consists of absorbent paper,
nitrocellulose membrane, sample pad, adhesive pad and rubber sheet.
T line (test line) of nitrocellulose membrane is coated with
2019nCOV antibody, and C line (quality control line) is coated with
goat anti-mouse polyclonal antibody, and the binding pad contains
labeled 2019nCOV antibody.
Sample buffer: phosphate, sodium azide, etc.
Storage Conditions and Validity
Keep it at 2℃~30℃, and the validity period is tentatively set at 18
months.
The validity period of aluminum foil bags is 1 hour.
Production batch number: see the label for details.
Expiry date: see the relevant contents of the label for details.
Sample Requirements
(1) nasal swab collection method: insert the sampling swab into the
nostril, and the tip of the swab should be inserted 2.5cm away from
the edge of the nostril. Roll the swab along the mucous membrane in
the nostril five times, and then repeat this process with the same
swab for the other nostril. (See Figure 1 for details)
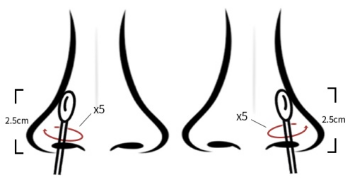
Figure 1 Collection method for nasal swab
(2) Sample treatment: the collected samples should be treated with
the sample buffer provided by this kit as soon as possible (if it
can't be treated immediately, the samples should be stored in a
dry, sterilized and tightly sealed container), stored at 2℃~8℃ for
no more than 24 hours, and stored at -70℃ for a long time (but
avoid repeated freezing and thawing).
Test Method
Please read the relevant instructions carefully before testing.
Please return all reagents to room temperature before testing, and
test at room temperature.
1. Sample treatment (see Figure 2)
1. Insert the sampled swab into the sample buffer and rotate it
around the inner wall for about 10 times to make the sample
dissolve in the solution as much as possible.
2. Squeeze the tip of the swab along the inner wall of the tube to
keep the liquid flowing into the tube as much as possible, and
remove and discard the swab.
3. Cover the dryer.

Figure 2 Sample processing

Figure 3 Detection procedure
2. Test procedure (see Figure 3)
1. take out the test card.
2. Add 2 drops (about 80μ50) to extract the processed sample into
the test card loading well, and then start the timer.
3. Read the results after 15 minutes at room temperature. After 20
minutes, the result is invalid
Interpretation of Test Results
Interpretation of test card (Figure 4):
① Invalid result: there is no reaction line on the quality control
line (line C), which is invalid and should be tested again.
② Negative result: the quality control line (line C), a red band,
is colored.
③ Positive results: Two red bands, the test line (T line) and the
quality control line (C line) are colored.

Figure 4 Interpretation of test results
Limitation of test method
1. This product is used for qualitative detection, only for
auxiliary diagnosis in vitro.
2. This product is suitable for nasal swab samples. Other sample
types may have inaccurate or invalid results.
3. Please make sure to add an appropriate number of samples for
testing. Too many or too few samples may lead to inaccurate
results.
4. The test results of this reagent are for clinical reference
only, and should not be used as the only basis for clinical
diagnosis and treatment. The final diagnosis of the disease should
be made after comprehensive evaluation of all clinical and
laboratory results.
Product Performance Indicators
1. detection limit: inactivated SARS-CoV-2 virus culture was used
in this study, and the minimum detection limit of the kit was
6×102TCID50/mL.
2. Test with the company's reference products, and the results
should meet the requirements of the company's reference products.
2.1 coincidence rate of positive reference products: the positive
reference products P1-P5 of the company are all positive.
2.2 The coincidence rate of negative reference materials: the
negative reference materials N1-N10 of enterprises are all
negative.
2.3 test limit: the lowest test limit reference product L1-L3 of
the enterprise is detected, L1 should be negative, L2 and L3 should
be positive.
2.4 Repeatability: the repeated reference products J1 and J2 of
enterprises were detected, and the results of each detection were
10 times positive.
3. Cross-reaction: Add the following concentrations of
microorganisms and viruses to the sample at the specified
concentration to evaluate their potential interference in the
2019-nCoVAg detection project. The results showed that there was no
cross reaction and no interference from various microorganisms and
viruses.
| SN | Microorganisms | Concentration | Cross reaction |
| 1 | Coronavirus (HKU1, OC43, NL63 and 229E) | 1.0×105TCID50/mL | No |
| 2 | H1N1 influenza (new type A H1N1 influenza virus (2009), seasonal
H1N1 influenza virus), H3N2, H5N1, H7N9 | 1.0×105TCID50/mL | No |
| 3 | Influenza B (Yamagata strain, Victoria strain) | 2.5×105TCID50/mL | No |
| 4 | Respiratory syncytial virus | 2.8×105TCID50/mL | No |
| 5 | Group A, B, C of rhinovirus | 2.0×105TCID50/mL | No |
| 6 | Type 1, 2, 3, 4, 5, 7, 55 of adenovirus | 2.0×105TCID50/mL | No |
| 7 | Group A, B, C and D of enterovirus | 2.0×105TCID50/mL | No |
| 8 | EB virus | 2.0×105TCID50/mL | No |
| 9 | Measles virus | 2.0×105TCID50/mL | No |
| 10 | Human cytomegalovirus | 2.0×105TCID50/mL | No |
| 11 | Rotavirus | 2.0×105TCID50/mL | No |
| 12 | Norovirus | 2.0×105TCID50/mL | No |
| 13 | Mumps virus | 2.0×105TCID50/mL | No |
| 14 | Varicella-zoster virus | 2.0×105TCID50/mL | No |
| 15 | Mycoplasma pneumoniae | 1.0×106CFU/mL | No |
| 16 | Legionella pneumophila | 1.0×106CFU/mL | No |
| 17 | Haemophilus influenzae | 1.0×106CFU/mL | No |
| 18 | Streptococcus pyogenes (group A) | 1.0×106CFU/mL | No |
| 19 | Streptococcus pneumoniae | 1.0×106CFU/mL | No |
| 20 | Escherichia Coli | 1.0×106CFU/mL | No |
| 21 | Pseudomonas aeruginosa | 1.0×106CFU/mL | No |
| 22 | Neisseria meningitidis | 1.0×106CFU/mL | No |
| 23 | Candida albicans | 1.0×106CFU/mL | No |
| 24 | Staphylococcus aureus | 1.0×106CFU/mL | No |
4. Interference substances: In 2019, the following concentrations
of drugs were added to the samples with specified concentrations to
evaluate their potential interference in nCoVAg detection items.
The results showed that all kinds of drugs did not interfere with
the detection results of this reagent.
| Interfering substances | Concentration | Interfering substances | Concentration |
| Mucoprotein | 1mg/mL | Ribavirin | 0.4mg/mL |
| Whole Blood | 1% | Fluticasone | 0.5mg/mL |
| Oxymetazoline | 10mg/mL | Dexamethasone | 5 mg/mL |
| Histamine hydrochloride | 10mg/mL | Triamcinolone acetonide | 5mg /mL |
| Tobramycin | 1mg/mL | Levofloxacin | 0.2 mg/mL |
| Oseltamivir | 1mg/mL | Azithromycin | 0.1 mg/mL |
| Zanamivir | 1mg/mL | Ceftriaxone | 0.4 mg/mL |
| Arbidol | 0.5mg/mL | Meropenem | 0.2 mg/mL |
5. Hook effect: After inactivated 2019-nCoV culture, no hook effect
was observed in the detection range of 1.0×106TCID50/mL with high
concentration.
6. Clinical study: RT-PCR reagent was used as contrast agent to
evaluate nasal swab samples. 120 positive samples and 120 negative
samples (RT-PCR detection) were selected for detection with
Zhongxiu detection reagent. The research results are summarized as
follows:
| Nasal swab | RT-PCR | Sum |
| Positive | Negative |
 | Positive | 116 | 2 | 118 |
| Negative | 4 | 118 | 122 |
| Sum | 120 | 120 | 240 |
| Sensitivity | 96.67%, (95%CI: 91.74%~98.70%) |
| Specificity | 98.33%, (95%CI: 94.13%~99.54%) |
Precautions
1. This product is only used for in vitro diagnosis.
2. This product is disposable and cannot be reused.
3. Read the operating instructions carefully before operation, and
conduct experimental operation in strict accordance with the
reagent operating instructions.
4. Avoid conducting experiments under harsh environmental
conditions (including 84 kinds of disinfectants, sodium
hypochlorite, acid and alkali, acetaldehyde and other
high-concentration corrosive gases and dust). After the experiment,
the laboratory should be disinfected.
5. All samples and reagents used should be regarded as potentially
infectious substances and treated according to local regulations.
6. Reagent should be used within the validity period indicated on
the outer package. It should be used as soon as possible after
being taken out of the aluminum foil bag to prevent moisture.
Logo interpretation
 | Do not re-use |  | Store at 2℃~30℃ |
 | Consult instructions for use |  | In vitro diagnostic medical devic |
 | Batch code |  | Use-by date |
 | Keep dry |  | Keep away from sunlight |
 | Authorized representative in the European Community |  | Manufacturer |
Basic Information
 | ZHONGXIU SCIENCE AND TECHNOLOGY CO.,LTD Dingluan industrial zone ,Changyuan City,453400,P.R.CHINA Tel:+86-371-55016575 Email:zosbio@zosbio.com |
 | SUNGO Europe B.V. Olympisch Stadion 24, 1076DE Amsterdam, Netherlands
|
Company profile
Zhongxiu Technology Co., Ltd. is a high-tech enterprise
specializing in the research, development, production and
management of in vitro diagnostic products. In vitro diagnostic
products developed by the company include POCT series, microbial
series, biochemical series, immune series reagents and supporting
instruments.
The company always adheres to the core concept of "speed, accuracy
and life", and is committed to providing high-quality products and
services for the society and contributing to human health.