2019-NCoV Ag Rapid Test (Immunochromatography) Usage Manual For
Self-Test Test Method
Intended Use
The kit is used for qualitative detection of ncov-Ag collected from
human nasal swab samples in 2019.
As a new weapon, ncov in 2019 is a new weapon β-Joe. It can cause
viral pneumonia, and its main clinical manifestations are fever,
fatigue and dry cough. A few patients suffer from nasal congestion,
runny nose, sore throat, diarrhea and other symptoms. Critically
ill patients usually develop dyspnea and/or hypoxemia after one
week. In severe cases, it can rapidly develop into acute
respiratory distress syndrome, septic shock, refractory metabolic
acidosis and coagulation disorder.
The 2019 ncovag strains in nasal swabs of suspicious patients were
detected by lateral flow immunoassay. In the acute stage of
infection, antigens are usually detected in nasal swab samples. A
positive result indicates the existence of virus antigen, but the
clinical correlation between the patient's medical history and
other diagnostic information is also a necessary condition for
determining the infection status. Positive results cannot rule out
bacterial infection or co-infection with other viruses.
This toolbox is used by laymen in non-laboratory environment (such
as personal residence or some non-traditional places, such as
offices, sports activities, airports, schools, etc.). The test
results of this kit are for clinical reference only. It is
suggested to make comprehensive analysis according to clinical
manifestations and other laboratory tests.
Principle of Detection
The kit is an immunoassay kit based on the principle of double
antibody sandwich technology. The 2019 ncov monoclonal antibody
labeled with markers was sprayed on the binding pad as an
indicator. In the test process, the 2019ncov-Ag in the sample
combined with the labeled 2019ncov monoclonal antibody to form an
Ag-AB complex. The complex migrated upward by capillary effect
until it was captured by another 2019 ncov monoclonal antibody,
forming a sandwich complex. If 2019 ncov-Ag exists in the sample, a
red band will appear in the T area of the interpretation window.
Otherwise, it will be a negative result. A control line (c) is used
for program control. If the test program is executed correctly, it
should always be displayed.
Main Components
The kit consists of a test card, a sample buffer and a swab.
Test card: It consists of aluminum foil bag, desiccant, test strip
and plastic card. The test strip consists of absorbent paper,
nitrocellulose membrane, sample pad, adhesive pad and rubber sheet.
T-line (test line) of nitrocellulose membrane was coated with
2019nCOVAb, C-line (quality control line) was coated with goat
anti-mouse polyclonal Ab, and 2019nCOVAb was attached to the pad.
Sample buffer: phosphate, sodium azide, etc.
Storage Conditions and Validity
Keep it at 2℃~30℃, and the validity period is tentatively set at 18
months.
The validity period of aluminum foil bags is 1 hour.
Production batch number: see the label for details.
Expiry date: see the relevant contents of the label for details.
Sample Requirements
(1) nasal swab collection method: insert the sampling swab into the
nostril, and insert the tip of the swab 2.5cm away from the edge of
the nostril. Roll the swab along the mucosa in the nostril five
times, and then repeat the process with the same swab (see Figure
1)
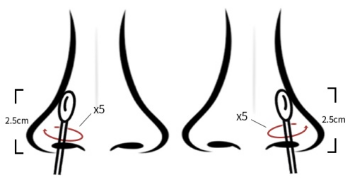
Figure 1 Collection method for nasal swab
(2) Sample treatment: the collected samples should be treated with
the sample buffer provided by this box as soon as possible (if they
can't be treated immediately, they should be stored in dry,
sterilized and sealed containers), and stored at -70℃ within 2
hours (but avoid repeated freezing and thawing)
Test Method
Please read the instructions carefully before testing. Please
return all reagents to room temperature before testing. The test
should be carried out at room temperature.
1. sample processing flow (see fig. 2)
1. Insert the sampling swab into the sample buffer and rotate it
close to the inner wall for about 10 times to make the sample
dissolve in the solution as much as possible.
2. Squeeze the tip of the swab along the inner wall of the tube to
make the liquid flow into the tube as much as possible, and take
out and discard the swab.
3. Cover the dryer.

Figure 2 Sample processing

Figure 3 Detection procedure
2. Test procedure (see Figure 3)
1. take out the test card.
2. Add 2 drops (about 80μ50) to extract the processed sample into
the test card loading well, and then start the timer.
3. Read the results after 15 minutes at room temperature. After 20
minutes, the result is invalid
Interpretation of Test Results
Test card description (figure 4):
① invalid result: the quality control line (line c) is invalid
without response line, and should be tested again.
② Negative line result: the quality control line (line C) is red
ribbon.
③ Positive results: The two red bands, test line (T line) and
quality control line (C line) are all colored.

Figure 4 Interpretation of test results
Limitation of test method
1. This product is used for qualitative detection, only for
auxiliary diagnosis in vitro.
2. This product is suitable for nasal swab samples. Results of
other sample types may be inaccurate or invalid.
3. Please make sure to add an appropriate number of samples for
testing. Excessive or small sample size may lead to inaccurate
results.
4. The test results of this reagent are for clinical reference
only, and should not be used as the only basis for clinical
diagnosis and treatment. The final diagnosis can only be made after
a comprehensive evaluation of all clinical and laboratory results.
Product Performance Indicators
1. detection limit: in this study, inactivated SARS-CoV-2 virus was
used for culture, and the minimum detection limit of the kit was
6×102TCID50/mL.
2. Use the company reference products for testing, and the results
should meet the requirements of the company reference products.
2.1 pass rate of positive control products: P1-P5 of positive
control products of the company are all positive.
2.2 pass rate of negative control products: N1-N10 of the company's
negative control products are all negative.
2.3 detection limit: the minimum detection limit reference products
L1-L3 are detected, L1 is negative, L2 and L3 are positive.
2.4 Reproducibility: The reference materials J1 and J2 were reused
by the test company, and each test was positive 10 times.
3. Cross-reaction: Add the following microorganisms and viruses to
the samples at the specified concentration to evaluate their
potential interference to the nCoVAg test items in 2019. The
results showed that there was no cross reaction and interference
from various microorganisms and viruses.
| SN | Microorganisms | Concentration | Cross reaction |
| 1 | Coronavirus (HKU1, OC43, NL63 and 229E) | 1.0×105TCID50/mL | No |
| 2 | H1N1 influenza (new type A H1N1 influenza virus (2009), seasonal
H1N1 influenza virus), H3N2, H5N1, H7N9 | 1.0×105TCID50/mL | No |
| 3 | Influenza B (Yamagata strain, Victoria strain) | 2.5×105TCID50/mL | No |
| 4 | Respiratory syncytial virus | 2.8×105TCID50/mL | No |
| 5 | Group A, B, C of rhinovirus | 2.0×105TCID50/mL | No |
| 6 | Type 1, 2, 3, 4, 5, 7, 55 of adenovirus | 2.0×105TCID50/mL | No |
| 7 | Group A, B, C and D of enterovirus | 2.0×105TCID50/mL | No |
| 8 | EB virus | 2.0×105TCID50/mL | No |
| 9 | Measles virus | 2.0×105TCID50/mL | No |
| 10 | Human cytomegalovirus | 2.0×105TCID50/mL | No |
| 11 | Rotavirus | 2.0×105TCID50/mL | No |
| 12 | Norovirus | 2.0×105TCID50/mL | No |
| 13 | Mumps virus | 2.0×105TCID50/mL | No |
| 14 | Varicella-zoster virus | 2.0×105TCID50/mL | No |
| 15 | Mycoplasma pneumoniae | 1.0×106CFU/mL | No |
| 16 | Legionella pneumophila | 1.0×106CFU/mL | No |
| 17 | Haemophilus influenzae | 1.0×106CFU/mL | No |
| 18 | Streptococcus pyogenes (group A) | 1.0×106CFU/mL | No |
| 19 | Streptococcus pneumoniae | 1.0×106CFU/mL | No |
| 20 | Escherichia Coli | 1.0×106CFU/mL | No |
| 21 | Pseudomonas aeruginosa | 1.0×106CFU/mL | No |
| 22 | Neisseria meningitidis | 1.0×106CFU/mL | No |
| 23 | Candida albicans | 1.0×106CFU/mL | No |
| 24 | Staphylococcus aureus | 1.0×106CFU/mL | No |
4. Interference substances: In 2019 nCoV-Ag test project, the
following concentrations of drugs were added to samples to evaluate
their potential interference. The results showed that all kinds of
drugs did not interfere with the detection results of reagents.
| Interfering substances | Concentration | Interfering substances | Concentration |
| Mucoprotein | 1mg/mL | Ribavirin | 0.4mg/mL |
| Whole Blood | 1% | Fluticasone | 0.5mg/mL |
| Oxymetazoline | 10mg/mL | Dexamethasone | 5 mg/mL |
| Histamine hydrochloride | 10mg/mL | Triamcinolone acetonide | 5mg /mL |
| Tobramycin | 1mg/mL | Levofloxacin | 0.2 mg/mL |
| Oseltamivir | 1mg/mL | Azithromycin | 0.1 mg/mL |
| Zanamivir | 1mg/mL | Ceftriaxone | 0.4 mg/mL |
| Arbidol | 0.5mg/mL | Meropenem | 0.2 mg/mL |
5. Hook effect: In the high concentration range of 1.0,
×106TCID50/mL, 2019nCoV inactivated culture was not observed.
6. Clinical research: Using RT-PCR detection reagent as contrast
agent to evaluate nasal swab specimens. Choose 120 positive and 120
negative (RT-PCR detection), and use Zhongxiu reagent for
detection. The research results are summarized as follows:
| Nasal swab | RT-PCR | Sum |
| Positive | Negative |
 | Positive | 116 | 2 | 118 |
| Negative | 4 | 118 | 122 |
| Sum | 120 | 120 | 240 |
| Sensitivity | 96.67%, (95%CI: 91.74%~98.70%) |
| Specificity | 98.33%, (95%CI: 94.13%~99.54%) |
Precautions
1. This product is only used for in vitro diagnosis.
2. This product is disposable and cannot be recycled.
3. Read the instructions carefully before operation, and carry out
experimental operation in strict accordance with the reagent
instructions.
4. Avoid harsh environmental conditions (including 84
disinfectants, sodium hypochlorite, acid and alkali or
acetaldehyde, and other high-concentration corrosive gases and
dust, etc.). After the experiment, the laboratory should be
disinfected.
5. All samples and reagents used should be regarded as potentially
infectious materials and treated according to local laws and
regulations.
6. The reagent should be used within the validity period indicated
on the outer packaging. After taking it out of the aluminum foil
bag, use the test card as soon as possible to prevent moisture.
Logo interpretation
 | Do not re-use | 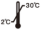 | Store at 2℃~30℃ |
 | Consult instructions for use |  | In vitro diagnostic medical devic |
 | Batch code |  | Use-by date |
 | Keep dry |  | Keep away from sunlight |
 | Authorized representative in the European Community |  | Manufacturer |
Basic Information
 | ZHONGXIU SCIENCE AND TECHNOLOGY CO.,LTD Dingluan industrial zone ,Changyuan City,453400,P.R.CHINA Tel:+86-371-55016575 Email:zosbio@zosbio.com |
 | SUNGO Europe B.V. Olympisch Stadion 24, 1076DE Amsterdam, Netherlands
|
Company profile
Zhongxiu Science And Technology Co.,Ltd., is a high-tech enterprise
engaged in the research and development, production and operation
of in vitro diagnostic products. The in vitro diagnostic products
developed by the company cover POCT series, microbial series,
biochemical series and immune series reagents and supporting
instruments.
The company has always adhered to the core concept of "fast and
accurate, living up to life", committed to providing society with
excellent products and services, and contributing to the cause of
human health.