BfArm Whole Blood Antigen Test Nasal Swab Self Test At Home Useful
Intended Use
Kkit was purchased through the qualitative detection of ncov AG.
Nasal specimens were collected
As a new weapon, the ncov in 2019 is β The main clinical symptoms
of viral pneumonia are fever, fatigue and cough. Many patients will
have a straight nose, runny nose, sore throat or diarrhea. Severe
patients usually develop dyspnea or hypoxemia after one week.
Severe cases will soon develop into acute dyspnea syndrome, septic
shock, metabolic poisoning of my teeth, and blood disorders.
Through the Deputy immune examiner, the suspected patient is
ncovag. It was detected in 2019. The antigen was detected in the
nose experiment. Benign textures and viral antigens are essential
to confirm the clinical link between the patient's medical record
and other diagnostic information. Benign textures are associated
with bacterial infections or other viruses.
Remen is located in a non residential environment (e.g. private
homes and offices, sports activities, airports, schools). Provide
the test results and clinical reference of this kit. Comprehensive
analysis of clinical symptoms and other prosecutors.
Principle of Detection
This is a drug based on antibody adjuvant. The 2019ncov monoclonal
antibody table was configured on the binding pad. In the test
project, combined with the label test data, the Ag 19cov monoclonal
antibody and Ag AB complex were captured by other 199ncov
monoclonal antibodies and moved upward through the capillary
phenomenon as a sandwich complex. If 2019 NCO vag is present in the
sample, the red tape will be displayed in the T area of the window.
In addition, the results are negative. The control line (c) is used
for program control. If the test program works normally, please
keep it displayed.
Main Components
Wipe the reagent ruler with test card, sample bag or cloth.
Test card: it is composed of aluminum foil, desiccant, test cream
and plastic card. The test paper is composed of absorption paper,
nickel filiform film, test pad, adhesive pad and rubber pad.
2019nvab (the test line is covered with t line of nitrocellulose
film) and goat mouse clone line AB (quality control line) are
covered with C line and 2019nvab. On the mat.
Sample buffer: phosphoric acid, sodium nitride, etc.
Storage Conditions and Validity
Keep it at 2 ℃ ~ 30 ℃, and the validity period is tentatively 18
months.
The aluminum foil strip can be used for 1 hour.
Production batch number: see the table for details.
Please refer to talabel for details.
Sample Requirements
(1) Collect the tip of the nose: wipe the sample, insert it into
the nasal cavity, and shake it with the tip of the nose for 2.5cm.
Repeat bending # 5 with the same rag along the nasal mucosa (see
Figure 1)
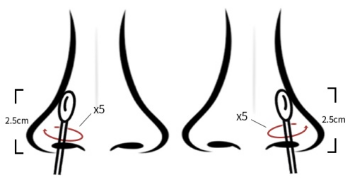
Figure 1 Collection method for nasal swab
(2) Sample processing: the recovered samples shall be processed as
soon as possible in the sample buffer prepared in the box (do not
process immediately, but store in a dry and sterilized sealed
container) - 702 hours. C (to avoid freezing)
Test Method
Please read the instructions carefully before testing. All reagents
were determined at room temperature. Room temperature test
(1) Test material treatment technology (see Figure 2)
Insert the sample exchange solution into the sample buffer and
rotate it about 10 times to the inner wall to make the sample
dissolve in the solution as much as possible.
1. Inject liquid into the pipe in front of the inner wall of the
pipe and pour it out.
2. Cover with a hair dryer.

Figure 2 Sample processing

Figure 3 Detection procedure
(2) Review sequence (refer to figure 3)
1. Take out the test card.
2. 2 drops and μ 50) place the test material on the test card to
stop Tingmei from working.
Read the texture after 15 minutes at room temperature. Invalid
results after 5 minutes
Interpretation of Test Results
Test card description (Figure 4)
Invalid ① Result: the quality management line (line C) is invalid.
If there is no answer line, it needs to be confirmed again.
② Negative result: the quality management line (c) is a red ribbon.
③ Front hand feel, all colors of two red bands: knife side line (T)
and quality management line (c).

Figure 4 Interpretation of test results
Limitation of test method
1. This product is only used for invitro auxiliary diagnosis of
qualitative inspector.
2. This product is suitable for the shape of the nose. Other sample
types are textures and may be negative or invalid.
3. Test samples increased significantly. Because the sample is too
large or too small, the result may be inaccurate.
4. It cannot be the only basis for clinical trials and treatment.
The final diagnosis can only be made through a comprehensive
evaluation of all clinical and experimental textures.
Product Performance Indicators
1. Detection limit: the minimum detection limit is 6 × Inactivated
sars-cov-2 virus with 102tid 50 / ml
2. The test results of the company's reference products must meet
the requirements of the company's reference products.
2.1 control the access rate of the product. p. 1 - p. 15.
Enterprises are indeed managing products.
2.2 multiple products: enterprise voice control products n1-n10 are
voice.
2.3 test: the minimum test of L1-L3 and L1 of control products is
negative, and L2 and L3 are positive.
2.4 repeatability: reference materials J1 and J2 have been
repeatedly used for 10 times in swordcompany.
3. Cross reactivity: in order to evaluate the potential hazards of
nco-vag test project in 2019, the following microorganisms and
bares were added to the samples with fixed concentration,
indicating that various microorganisms and viruses have no cross
reactivity and interference.
| SN | Microorganisms | Concentration | Cross reaction |
| 1 | Coronavirus (HKU1, OC43, NL63 and 229E) | 1.0×105TCID50/mL | No |
| 2 | H1N1 influenza (new type A H1N1 influenza virus (2009), seasonal
H1N1 influenza virus), H3N2, H5N1, H7N9 | 1.0×105TCID50/mL | No |
| 3 | Influenza B (Yamagata strain, Victoria strain) | 2.5×105TCID50/mL | No |
| 4 | Respiratory syncytial virus | 2.8×105TCID50/mL | No |
| 5 | Group A, B, C of rhinovirus | 2.0×105TCID50/mL | No |
| 6 | Type 1, 2, 3, 4, 5, 7, 55 of adenovirus | 2.0×105TCID50/mL | No |
| 7 | Group A, B, C and D of enterovirus | 2.0×105TCID50/mL | No |
| 8 | EB virus | 2.0×105TCID50/mL | No |
| 9 | Measles virus | 2.0×105TCID50/mL | No |
| 10 | Human cytomegalovirus | 2.0×105TCID50/mL | No |
| 11 | Rotavirus | 2.0×105TCID50/mL | No |
| 12 | Norovirus | 2.0×105TCID50/mL | No |
| 13 | Mumps virus | 2.0×105TCID50/mL | No |
| 14 | Varicella-zoster virus | 2.0×105TCID50/mL | No |
| 15 | Mycoplasma pneumoniae | 1.0×106CFU/mL | No |
| 16 | Legionella pneumophila | 1.0×106CFU/mL | No |
| 17 | Haemophilus influenzae | 1.0×106CFU/mL | No |
| 18 | Streptococcus pyogenes (group A) | 1.0×106CFU/mL | No |
| 19 | Streptococcus pneumoniae | 1.0×106CFU/mL | No |
| 20 | Escherichia Coli | 1.0×106CFU/mL | No |
| 21 | Pseudomonas aeruginosa | 1.0×106CFU/mL | No |
| 22 | Neisseria meningitidis | 1.0×106CFU/mL | No |
| 23 | Candida albicans | 1.0×106CFU/mL | No |
| 24 | Staphylococcus aureus | 1.0×106CFU/mL | No |
4. Interference: in the nco-vag trial in 2019, in order to evaluate
the potential hazards, it is planned to add the following
concentrations of drugs to the sample. The results showed that all
agents did not hinder the reagent test results.
| Interfering substances | Concentration | Interfering substances | Concentration |
| Mucoprotein | 1mg/mL | Ribavirin | 0.4mg/mL |
| Whole Blood | 1% | Fluticasone | 0.5mg/mL |
| Oxymetazoline | 10mg/mL | Dexamethasone | 5 mg/mL |
| Histamine hydrochloride | 10mg/mL | Triamcinolone acetonide | 5mg /mL |
| Tobramycin | 1mg/mL | Levofloxacin | 0.2 mg/mL |
| Oseltamivir | 1mg/mL | Azithromycin | 0.1 mg/mL |
| Zanamivir | 1mg/mL | Ceftriaxone | 0.4 mg/mL |
| Arbidol | 0.5mg/mL | Meropenem | 0.2 mg/mL |
5. Hook effect: within the high concentration range of 1.0. No. ×
106tcid 50 / ml, 19ncov no inert culture was found.
6. The clinical expansion area was evaluated by heavy water reagent
120 positive and 120 negative (RT-PCR side). The texture is shown
below.
| Nasal swab | RT-PCR | Sum |
| Positive | Negative |
 | Positive | 116 | 2 | 118 |
| Negative | 4 | 118 | 122 |
| Sum | 120 | 120 | 240 |
| Sensitivity | 96.67%, (95%CI: 91.74%~98.70%) |
| Specificity | 98.33%, (95%CI: 94.13%~99.54%) |
Precautions
1. This product can only be used for invitro diagnosis.
2. This product is disposable and cannot be reused. Please read the
instructions carefully before operation.
3. Strictly follow the instructions of the reagent.
4. The laboratory shall avoid disinfection of 84 kinds of corrosive
gases and dust with high concentration, such as sodium
hypochlorite, acid-base, aldehyde, etc.
5. All used samples and reagents are considered as substances that
may be infected and must be treated in accordance with local laws.
6. Please use the reagent indicated on the external package within
the validity period. Please take out the test card from the
aluminum foil pocket for use.
Logo interpretation
 | Do not re-use | 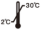 | Store at 2℃~30℃ |
 | Consult instructions for use |  | In vitro diagnostic medical devic |
 | Batch code |  | Use-by date |
 | Keep dry |  | Keep away from sunlight |
 | Authorized representative in the European Community |  | Manufacturer |
Basic Information
 | ZHONGXIU SCIENCE AND TECHNOLOGY CO.,LTD Dingluan industrial zone ,Changyuan City,453400,P.R.CHINA Tel:+86-371-55016575 Email:zosbio@zosbio.com Web:www.zosbio.com |
 | SUNGO Europe B.V. Olympisch Stadion 24, 1076DE Amsterdam, Netherlands
|
Company profile
Zhongxiu Science And Technology Co.,Ltd., is a high-tech enterprise
engaged in the research and development, production and operation
of in vitro diagnostic products. The in vitro diagnostic products
developed by the company cover POCT series, microbial series,
biochemical series and immune series reagents and supporting
instruments.
The company has always adhered to the core concept of "fast and
accurate, living up to life", committed to providing society with
excellent products and services, and contributing to the cause of
human health.
















