Sample Requirements
(1) Saliva sample collection: Do not eat or drink chewing gum or
tobacco products within 30 minutes after saliva sample collection.
Place the tip of the tongue on the palate to collect saliva roots.
Disposable sterile cotton swabs are placed under saliva samples.
Use the tip of the tongue for at least 10 seconds, completely
immerse in saliva and rotate for more than 5 times. (see table 1)
Note: If the saliva sample is not collected correctly.
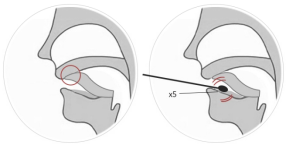
Figure 1 Methodsfor saliva sample collection

Figure 2 Methods for sputum sample collection
(2) Sputum specimen collection: rinse with water, cough up sputum,
put it into specimen bag, and wipe the specimen with disposable
sterile cotton swabs. (see figure 2)
(3) Sample treatment: use the sample buffer provided in this kit to
treat the collected samples (samples that are not immediately
treated should be stored in dry, sterile and sealed containers).
2-8 degrees Celsius to 24 hours, more than 70 degrees Celsius (but
avoid repeated freezing and thawing).
Test Method
Please read the instruction carefully before testing. Return all
reagents to room temperature and test at room temperature.
Example processing (see Figure 3).
(1) Insert the sample swab into the sample buffer, rotate the
sample near the inner wall for about 10 times, and dissolve the
sample in the solution as much as possible.
(2) Press the swab head along the inner wall to make the liquid
flow into the pipeline, take out the swab and discard it.
(3) Cover with small water droplets.

Figure 3 Sample processing

Figure 4 Test procedure
2. Test process (see Figure 4).
(1) take out the test card.
(2) Add 2 drops (~80L) of processed sample extract to the sample
well of the test card, and start timing.
(3) Read the card at room temperature for 15 minutes, and the
result is invalid after 20 minutes.
Interpretation of Test Results
Test card description (see Figure 5):
1. Result: The quality control line (line C) should be rechecked.
2. Negative results: quality control line (C line), red ribbon and
coloring.
3. Positive results: There are two red stripes on the detection
line (T line) and the quality control line (C line).

Figure 5 Interpretation of test results
Limitation of Test Method
1. This product is a qualitative test, which is only used to assist
in vitro diagnosis.
2. This product is suitable for saliva and sputum samples, and
other types of samples may have inaccurate or invalid sample
results.
3. If the patient has no sputum samples, nasopharyngeal swabs
should be used for detection.
4. Make sure to add the right amount of samples for testing. Or too
many samples may lead to inaccurate results.
5. The test results of this reagent are for clinical reference
only, and should not be used as the only basis for clinical
diagnosis and treatment. Comprehensive evaluation of all clinical
and laboratory results will finally confirm the disease.
Product Performance Indicators
1. detection limit: inactivated SARS-COV-2 virus culture was used
in this study. The lowest detection limit is 6102 tcid50/ml.
2. The test adopts company standard materials, and the test results
should meet the requirements of company standard materials.
2.1 active control pass rate: enterprises actively control p1-p5.
2.2 Negative control pass rate: Our n1-n10 negative control test
was negative.
2.3 detection limit: refer to L1-L3, L1 is negative, L2 and L3 are
positive.
2.4 Reproducibility: J1 and J2 are 10 times positive.
3 Cross-reaction: Inject the following microorganisms and viruses
into specific concentration samples to evaluate their potential
interference with the 2019 novel coronavirus antigen detection
procedures.
4. Interference: Evaluate the potential interference in the 2019
nCoV Ag detection procedure, and add the following drugs at the
specified concentration. The results showed that all kinds of drugs
did not interfere with the detection results of reagents.
5. Hook effect: There is no hook effect in the high concentration
range of 1.0106 TCID50/mL.
6. Clinical study: Using RT-PCR sputum reagent as control reagent
to detect saliva and sputum samples respectively. For each sample
size (RT-PCR test), 120 positive and negative samples were
selected, and the second test was conducted with XIUS reagent. The
survey results are summarized as follows:
Precautions
1. This product is used for in vitro diagnosis.
2. This product is disposable and cannot be recycled.
3. Please strictly abide by the reagent instructions, and please
read the instructions carefully before the experiment.
4. Avoid testing under harsh environmental conditions (including
high-concentration corrosive gases, such as 84 disinfectant, dust,
sodium hypochlorite, acid and alkali, acetaldehyde, etc.).
Laboratory disinfection should be carried out after the experiment.
Company profile