Sample Requirements
(1) Saliva sampling: do not eat or drink chewing gum or cigarette
products within 30 minutes after saliva sampling. Place the tip of
the tongue on the mouth lid to collect the roots of saliva. Place a
disposable disinfecting swab under the saliva sample. Use the tip
of your tongue for at least 10 seconds, soaking it completely in
saliva and rotating it more than 5 times. (See Table 1)
Note: Saliva samples were collected incorrectly.
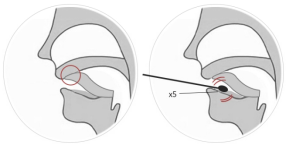
Figure 1 Methodsfor saliva sample collection

Figure 2 Methods for sputum sample collection
(2) Sputum collection: wash the sputum with water, put the sputum
into the test bag, and wipe the sputum with a disposable sterile
cotton stick. (Refer to Figure 2)
(3) Sample processing: Use the sample buffer storage contained in
the supporting components to process the collected samples (samples
that are not immediately discarded must be stored in a dry and
germicidal sealed container). 2-8 degrees Celsius to 24 hours,
above 70 degrees Celsius (however, please avoid repeated freezing
and thawing).
Test Method
Please read the instructions carefully before the test. All
reagents are returned to room temperature and tested at room
temperature.
Processing example (see Figure 3).
(1) Insert the sample exchanger into the sample buffer memory,
rotate it around the inner wall for about 10 times, and dissolve
the sample in solution as much as possible.
(2) Press the casing head along the inner wall to make the liquid
flow into the pipe, remove the casing and discard it.
(3) Cover with small water drops.

Figure 3 Sample processing

Figure 4 Test procedure
2. Test process (see Figure 4).
(1) Remove the test card.
(2) Add 2 drops (~ 80L) of treated sample extract to the sample
well of the test card and start timing.
(3) Read the card for 15 minutes at room temperature, and the
result is invalid after 20 minutes.
Interpretation of Test Results
Test card description (refer to Figure 5) :
1. Results: The quality management line (C line) needs to be
checked again.
2. Negative result: Quality control line (C line), red ribbon,
coloring.
3. Positive results: There are two red stripes on the test line (T
line) and quality management line (C line).

Figure 5 Interpretation of test results
Limitation of Test Method
1. This product is a qualitative test intended only to support
INVitro diagnostics.
2. This product is suitable for saliva and sputum samples. Other
types of samples may be incorrect or invalid.
3. In the absence of sputum samples, patients should be examined
with a nasopharyngeal swan.
4. Please add a suitable amount of samples for testing. Or, if
there are too many samples, the results may be incorrect.
5. The test results of the reagent are for clinical reference only,
and should not be used as the sole basis for clinical diagnosis and
treatment. The combined evaluation of all clinical and examination
results leads to a definitive diagnosis of the disease.
Product Performance Indicators
1. Detection limits: Inactive SARS-CCOV-2 virus cultures were used
in this study. The minimum detection limit is 610TCID50 / mL.
2. The company's standard materials shall be used in the
examination, and the test results shall meet the requirements of
the company's standard materials.
2.1 Qualification rate of active management: p1-P5.
2.2 Negative control pass rate: N1-N10 Negative control test is
negative.
2.3 Test limit: please refer to L1-L3. L1 is negative, L2 and L3
are positive.
2.4 Reproducibility: J1 and J2 are positive 10 times.
3 cross-reactivity: The following microorganisms and viruses were
injected into the sample at specific concentrations to evaluate
potential interference with the 2019 new corona virus antigen
detection program.
4. Interference: the 2019nCoV Ag detection step evaluates potential
interference and adds the following agents at specified
concentrations. The results show that various drugs do not
interfere with the result of reagent detection.
5. Hook effect: There is no hook effect within the high
concentration range of 1.0106 TCID50/mL.
6. Clinical studies: The RT-PLR sputum reagent was used as a
control reagent to detect saliva and sputum samples separately. For
each sample size (RT-PLR test), 120 positive and negative samples
were selected for a second test using the XIUS reagent. The
findings are summarized below.
Precautions
1. This product is used for INVitro diagnosis.
This product is disposable and can't be reused.
3. Please strictly follow the instructions of reagents. Please read
the instructions carefully before the experiment.
4. Do not perform the test under severe environmental conditions
(including 84 disinfectants, dust, sodium hypochlorite, acid and
alkali, acetaldehyde and other corrosive gases with high
concentration). Disinfection of the laboratory must be carried out
after the experiment.
Company profile
Zhongxiu Science And Technology Co.,Ltd., is a high-tech enterprise
engaged in the research and development, production and operation
of in vitro diagnostic products. The in vitro diagnostic products
developed by the company cover POCT series, microbial series,
biochemical series and immune series reagents and supporting
instruments.
The company has always adhered to the core concept of "fast and
accurate, living up to life", committed to providing society with
excellent products and services, and contributing to the cause of
human health.